Coronavirus | Vaccine shot ‘painless’, say Covishield trial volunteers
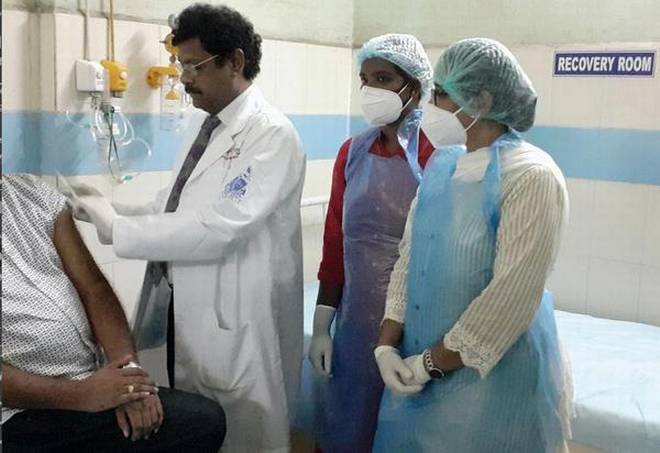
The vaccine COVISHIELD was administered to the first volunteer by the superintendent of KGH and principal of Andhra Medical College, Dr. PV Sudhakar. File | Photo Credit: Special arrangement
The trial involves 1,600 volunteers in 17 cities across the country
“It felt like a tetanus shot,” said Joel Joy, 32, who works for a multinational firm in Mumbai, and was among those who’d volunteered for the experimental Covishield vaccine, being tested by the Pune-based Serum Institute of India (SII) at various hospitals in India, “I'll be going to the gym later today,” he added.
The two-dose vaccine candidate is furthest ahead among the COVID-19 vaccines being tested in India. The trial involves 1,600 volunteers in 17 cities across the country likely to be inoculated in the coming months in the combined phase-2/3 trials. These trials check for whether the vaccine is safe and capable of producing an immune response to disarm future infections by the SARS-CoV-2 virus.
According to details of the trial available on the Indian Council of Medical Research (ICMR) site, the volunteers will be divided into two groups of 400 and 1,200 respectively with some in each group getting Covishield, some a placebo and yet others getting the Oxford/ChAdOx1. Both ‘Covishield’ and the Oxford/ChAdOx1 have the same virus protein but differ in their make-up — the exact nature of the difference has not been publicly disclosed. The trial also seeks to quantify these differences if any.
Social activist and Mumbai-based entrepreneur, Anil Hebbar, 56, also among the volunteers for the trial at the KEM hospital in Mumbai, said that after the vaccine, he felt nothing out of the ordinary other than a slight weariness.
“It was painless. I usually sleep after 1 a.m. and am up by 6 a.m. and travel extensively through the day and never feel particularly tired. But post the injection in the past four days, I feel a little extra tired,” he told The Hindu.
Being a double-blinded trial, neither the doctors nor the volunteers know who are the ones vaccinated and who've been given the placebo. The four volunteers who The Hindu spoke to, said they would be going for a follow-up shot on the 29th day.
Each of the inoculates involves 0.5 ml of the test-medicine being administered in a four-week interval. After a jab, a day’s dose of paracetamol is given to keep possible fever in check.
None of the participants said they registered a fever or a rash or itchiness at the site of the inoculation (the upper fore-arm).
“It was an ordinary experience and I was done in 30 minutes,” said Vaishali Janardhanan, 27, a volunteer and a social activist. “I was scrolling my phone during the shot. There were three women doctors on standby and because I was a woman, the 'pre-screening' involved a pregnancy test,” she said.
While anyone above 18 can volunteer, those who end up being part of a trial are pre-screened for a previous COVID infection as well as have their vitals examined.
At least one person, who was part of the Oxford vaccine trial in the United Kingdom and was administered the vaccine, suffered transverse myelitis, or an inflammation of the spinal cord due to which the trials were paused globally for reviews by safety boards. Trials have since resumed. All of the volunteers who spoke to The Hindu said they were made aware of these risks but were unperturbed.
“My parents were a little sceptical but I had no doubt. With COVID it’s now a matter of WHEN you will be infected rather than IF, given the spread we are seeing. Volunteering is the bare minimum I can do to help the world get a vaccine,” said Ms. Janardhanan.
Mr. Hebbar, Mr. Joy and Ms. Janardhanan said they were regularly involved in relief work and in organising supplies, donating blood and saw participation in the trial as an extension of what they always did.
Dr Rahul Ghule, a paediatrician, who was also vaccinated, said he planned to go for an antibody test in a month. Assuming the vaccine works as intended and a person didn't get a placebo, protective antibodies in response to the virus are expected to emerge in a month. However, it's still uncertain how long they would last and realistically confer protection.
Source - The Hindu
ABOUT US
OUR COMPANIES
CORPORATE SOCIAL RESPONSIBILITY
© Copyright 2025. Cyrus Poonawalla Group. All rights reserved.