India's Wait Over, Drug Regulator Says Covid Vaccines Cleared "110% Safe"
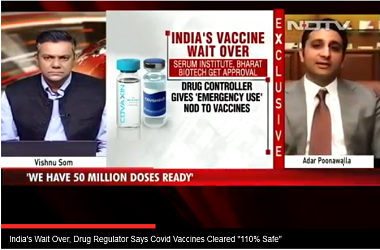
Drug Controller General of India VG Somani said,."We'll never approve anything if there is slightest of safety concern. The vaccines are 110 per cent safe".
New Delhi: Two vaccines for coronavirus, Oxford Institute's Covishield, which is being developed by the Pune-based Serum Institute, and Bharat Biotech's Covaxin, received emergency approval from the country's drug regulator on Sunday. "We'll never approve anything if there is slightest of safety concern. The vaccines are 110 per cent safe," Drug Controller General of India VG Somani said, adding Covishield was found to be 70.42 per cent effective and Bharat Biotech's Covaxin was "safe and provides a robust immune response". Hailing the scientific community and frontline Corona warriors, Prime Minister Narendra Modi tweeted, "It would make every Indian proud that the two vaccines that have been given emergency use approval are made in India". There is no word yet on when the vaccination process will begin.
Here are the top 10 points in this big story:
- "We'll never approve anything if there is slightest of safety concern. The vaccines are 110 per cent safe. Some side effects like mild fever, pain and allergy are common for every vaccine," Drug Controller General of India VG Somani said. The approval from the Drug Controller comes days after a government-appointed experts' panel gave clearance to the vaccines.
- Both vaccines have to be administered in two doses and stored at temperatures between 2 and 8 degrees Celsius. The government will give priority to 1 crore healthcare workers and 2 crore frontline workers when the vaccinations begin, Union Health Minister Dr Harshvardhan said as a countrywide dry run for the vaccination process was conducted on Saturday.
- "It would make every Indian proud that the two vaccines that have been given emergency use approval are made in India! This shows the eagerness of our scientific community to fulfil the dream of an Aatmanirbhar Bharat, at the root of which is care and compassion," PM Modi tweeted.
- Pune-based Serum Institute, the Drug Controller General said, conducted Phase 2 and Phase 3 trials on 1,600 participants in India. Recommendation was made for restricted use and the trials will continue, he added. The vaccine, developed by the Oxford University and pharma giant AstraZenca is already in use abroad.
- Bharat Biotech's Covaxin is conducting trials in collaboration with the Indian Council of Medical Research. The Drug Controller said that its Phase I and Phase II trials were conducted in around 800 people and the results showed that it is "safe and provides a robust immune response". The Phase III trial in on and 22,500 of the 25,800 participants have been vaccinated.
- The health ministry said the government's expert committee has reviewed Bharat Biotech's data on "safety and immunogenicity" and gave permission for "restricted use in emergency situation in public interest". The idea was to have "more options for vaccinations, especially in case of infection by mutant strains," the ministry said, adding that the clinical trials will continue.
- "Happy new year, everyone! All the risks @SerumInstIndia took with stockpiling the vaccine, have finally paid off. COVISHIELD, India's first COVID-19 vaccine is approved, safe, effective and ready to roll-out in the coming weeks," Serum Institute chief Adar Poonawalla tweeted.
- "It has been learnt that the vaccines of Bharat Biotech and the Serum Institute have received emergency approval. All preparations are underway for the Delhi government. First health workers and frontline workers will be given the vaccine, Then those above age 50 will be given the vaccine. Health workers and frontline workers will be vaccinated under First phase," Delhi health minister Satyendar Jain said. The vaccines will be given free of cost in Delhi, the minister earlier said.
- Flagging concerns over Bharat Biotech's Covaxin, senior Congress leader Shashi Tharoor tweeted, "The Covaxin has not yet had Phase 3 trials. Approval was premature and could be dangerous. @drharshvardhan should please clarify. Its use should be avoided till full trials are over. India can start with the AstraZeneca vaccine in the meantime".
- India has reported 18,177 new infections in the last 24 hours - 4.7 per cent lower than yesterday - taking the total Coronavirus cases to 1,03,23,965. Data from the health ministry showed the country has also logged 217 deaths, taking the total number of fatalities to 1,49435.
Source - The Economic Times
ABOUT US
OUR COMPANIES
CORPORATE SOCIAL RESPONSIBILITY
© Copyrights 2026. Cyrus Poonawalla Group. All rights reserved.