Oxford COVID-19 vaccine final trials will be held in these 17 hospitals in India
A total of 1,600 candidates will take part in the study.
King Edward Memorial Hospital Parel, and BYL Nair Hospitals, Mumbai Central — have received approval from ICMR to start the trial.
Three covid vaccines are undergoing trials in India
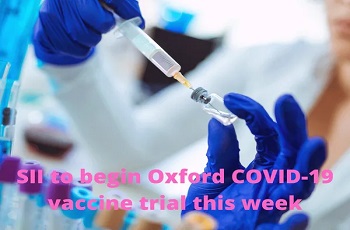
The phase II and phase III clinical trial of COVID-19 vaccine developed by the University of Oxford is all set to start in India. Indian pharmaceutical major Serum Institute of India has initiated the process, according to report by news agency ANI.
The COVID-19 vaccine was developed by the Jenner Institute, a part of the Nuffield Department of Medicine at the University of Oxford. The formulation is backed by by AstraZeneca PLC, a British-Swedish pharmaceutical company. AstraZeneca joined hands with Indian vaccine maker Serum Institute of India to manufacture COVID-19 vaccine for low-and-middle income countries.
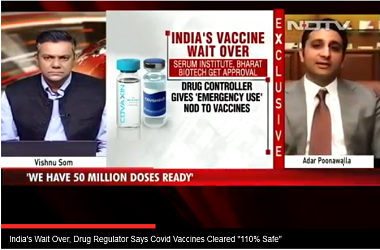
Earlier this month, Drugs Controller General of India gave nod to conduct advanced trials in India. "Phase 2/3 clinical trial will be observer-blind, randomised, controlled study to determine the safety and immunogenicity of Covishield (COVID-19 vaccine) in healthy Indian adults," according to the study design.
Pune based drug maker has selected 17 sites in India to conduct the trial. A total of 1,600 candidates will take part in the study.
These sites include — Andhra Medical College (Visakhapatnam), JSS Academy of Higher Education and Research, (Mysore), Seth G. S. Medical College and KEM Hospital (Mumbai), KEM Hospital Research Centre (Vadu), B J Medical College and Sassoon General Hospital (Pune), All India Institute Of Medical Sciences (Jodhpur), Rajendra Memorial Research Institute of Medical Sciences, (Patna), Institute of Community Medicine ( Madras), Post Graduate Institute of Medical Education & Research (PGIMER), Bharati Vidyapeeth Deemed University Medical College and Hospital (Pune), Jehangir Hospital ( Pune), AIIMS (Delhi), ICMR- Regional Medical Research Centre ( Gorakhpur), TN Medical College & BYL Nair Hospital (Mumbai), Mahatma Gandhi Institute of Medical Sciences (Sewagram) and Government Medical College (Nagpur).
Of 1,600 candidates, 400 participants will be part of the immunogenicity cohort and will be randomly assigned in a 3:1 ratio to receive either Covishield or Oxford/AZ-ChAdOx1 nCoV-19, respectively.The remaining 1,200 participants from safety cohort will be randomly assigned in a 3:1 ratio to receive either Covishield or Placebo, respectively, according to ANI.
Each participant will administer two doses in a gap of four weeks. First dose will be given on day one and second dose will be scheduled on day 29, according to the study design.
“After the completion of phase II trial, reports will be submitted to the Data Safety Monitoring Board, then to the Central Drugs Standard Control Organization for stage 3 of the trial," Dr Hemant Deshmukh, King Edward Memorial Hospital said.
Two civic-run hospitals in Mumbai — King Edward Memorial Hospital Parel, and BYL Nair Hospitals, Mumbai Central — have received approval from the Indian Council for Medical Research to start phase II and phase III clinical trial of COVID-19 vaccine developed by the University of Oxford. At present, Brihanmumbai Municipal Corporation (BMC) is looking for volunteers to participate in the trial.
Source - livemint
ABOUT US
OUR COMPANIES
CORPORATE SOCIAL RESPONSIBILITY
© Copyright 2026. Cyrus Poonawalla Group. All rights reserved.