Serum Institute’s Covishield vaccine may be recommended for international use
AstraZeneca is engaging with governments, multilateral organisations and other partners around the world for broad and equitable access to the vaccine without profit for the duration of the pandemic.
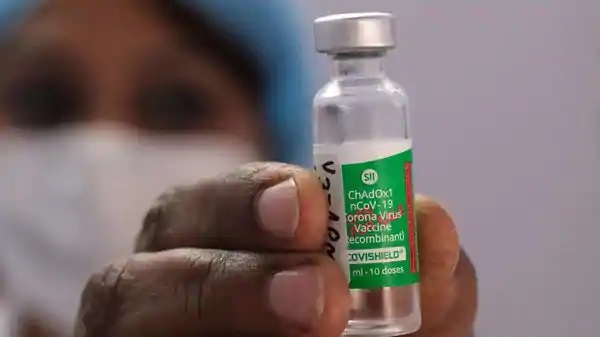
A hospital Staff member holds a vial of Covishield vaccine (Bloomberg)
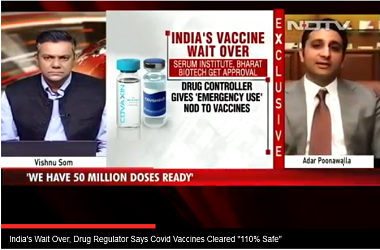
NEW DELHI : Pune-based Serum Institute of India’s (SII) Covishield vaccine developed by Oxford University and AstraZeneca, which recently got Indian drug regulator’s nod for use in covid-19 vaccination drive in India, may also get a green signal for global use from the World Health Organisation (WHO).
The global public health agency has indicated that it is looking forward to SII’s full data sets for rapid assessment to consider it for emergency use globally. “We look forward to Serum Institute of India submitting full data sets for rapid assessment so WHO can determine whether we can recommend their AstraZeneca vaccine for international use," said Tedros Adhanom Ghebreyesus, director general of the WHO during a press conference on Monday.
The WHO on December 31st 2020, listed the Comirnaty covid-19 mRNA vaccine for emergency use, making the Pfizer/BioNTech vaccine the first to receive emergency validation from the global health agency since the covid-19 outbreak. The WHO’s Emergency Use Listing (EUL) opens the door for countries to expedite their own regulatory approval processes to import and administer the vaccine. It also enables UNICEF and the Pan-American Health Organization to procure the vaccine for distribution to countries in need.
AstraZeneca has said that it is engaging with governments, multilateral organisations and other partners around the world for broad and equitable access to the vaccine without profit for the duration of the pandemic. “We have moved at pace during 2020 to build more than a dozen regional supply chains. The vaccine can be stored, transported and handled at normal refrigerated conditions (two-eight degrees Celsius/ 36-46 degrees Fahrenheit) for at least six months and administered within existing healthcare settings," Gagan Singh, Managing Director – AstraZeneca India told Mint.
Following the first authorisation for the vaccine by the UK Medicines and Healthcare products Regulatory Agency (MHRA) on 29 December 2020, oxford university-AstraZeneca vaccine has now seen approvals granted for emergency use in India as well as Argentina, Dominican Republic, El Salvador, Mexico and Morocco for the active immunisation of adults age 18 years or older.
“AstraZeneca has already submitted a substantial data package to support a conditional marketing authorisation for its covid-19 vaccine to the European Medicines Agency (EMA), as part of an ongoing rolling review process and will continue to work closely with the EMA to seek approval in the coming weeks," said Singh.
“AstraZeneca is also seeking Emergency Use Listing from the World Health Organization for an accelerated pathway to vaccine availability in low-income countries during this health crisis and has ongoing rolling reviews with many other regulatory authorities around the world," he added.
The Indian drug regulator reviewed the data generated from the AstraZeneca-University of Oxford clinical trial from the UK and Brazil before giving approval to the vaccine for use. The interim data for safety and immunogenicity of all participants from the SII's Phase 2/3 bridging trial in India was also reviewed.
Currently, SII has produced 50 million doses of Covishield. The pharmaceutical company’s capacity is 60-70 million doses per month, which will be increased further up to 100 million a month by February 2021. The Indian government on Monday signed a deal with SII to procure 11 million shots of the vaccine at ₹200 per dose.
Source - liveMint
ABOUT US
OUR COMPANIES
CORPORATE SOCIAL RESPONSIBILITY
© Copyrights 2026. Cyrus Poonawalla Group. All rights reserved.