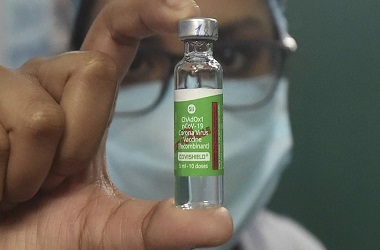
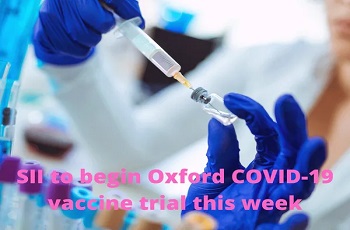
Oxford-SII's 'Covishield' likely to be Indias first COVID vaccine
Pune-based SII is to submit an updated safety data of phase 2 and 3 clinical trials in India, immunogenicity data from the clinical trial in the UK and India, as well as the assessment results of the UK Medicines and Healthcare products Regulatory Agency
With arrangements being made to possibly commence with inoculations starting next month in India, the drug regulator of the country is considering the vaccine candidate of Oxford University to initiate the vaccination drive.
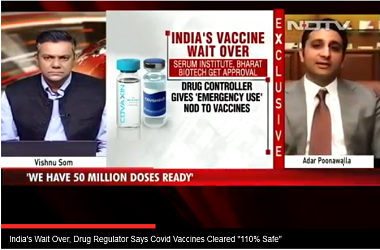
Before deciding to give the green light for emergency use authorisation to the vaccine being manufactured by the Serum Institute of India (SII), the Central Drugs Standard Control Organisation (CDSCO) is waiting for the UK to give the nod to the vaccine first.
Once the UK drug regulator gives its approval, which sources are expecting for to be sometime next week, the COVID-19 expert committee at the CDSCO will take over the proceedings for the country. The department is to hold a meeting to thoroughly review the clinical evaluations to ascertain the safety and immunogenicity standards of the vaccine before granting it emergency use authorisation, said official sources from the clinical evaluations conducted abroad and in India before granting any emergency authorisation for the vaccine here, official sources told PTI.
While Pfizer, whose vaccine candidate with German partner BioNtech has been granted approval for emergency use in over ten countries, the American pharma is yet to make a presentation in India. As far as the indigenous 'Covaxin' that is being manufactured by Bharat Biotech is concerned, emergency approval could take even more time owing to the fact that phase 3 clinical trials are still underway.
"Going by this, Oxford vaccine 'Covishield' is likely to be the first to be rolled out in India," said a source.
Last week, SII had submitted some additional requisite data to the Drug Controller General of India, added the sources. Earlier this month, SII and Bharat Biotech along with Pfizer had applied Pfizer had applied to the Drugs Controller General of India (DCGI) for emergency use authorisation for their vaccines. While the subject expert committee (SEC) on COVID-19 at CDSCO had asked for the former two companies for additional efficacy and safety data, Pfizer's application was not upheld as the company had asked for more time to come up with a presentation for the committee.
Amid fears about the mutated variant of SARS-CoV-2 detected in the UK, government officials recently said that it will have no impact on the potential of emerging vaccines that are being developed in India and other countries.
Pune-based SII is to submit an updated safety data of phase 2 and 3 clinical trials in India, immunogenicity data from the clinical trial in the UK and India, as well as the assessment results of the UK Medicines and Healthcare products Regulatory Agency (MHRA).
About Hyderabad-based Bharat Biotech, the SEC recommended that " the firm should present the safety and efficacy data from the ongoing phase 3 clinical trial in the country for further consideration."
Serum Institute of India happens to be the world's largest manufacturer of vaccines, and its collaboration with the University of Oxford and Astrazeneca has already led to 40 million doses being produced in the country.
Source - Business Today
ABOUT US
OUR COMPANIES
CORPORATE SOCIAL RESPONSIBILITY
© Copyright 2026. Cyrus Poonawalla Group. All rights reserved.