Serum Institute seeks approval to conduct local trial for Novavax Covid vaccine
US biotech firm Novavax has said that its Covid-19 vaccine was 89.3% effective in preventing the killer virus in a trial conducted in the United Kingdom
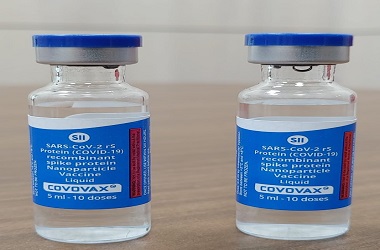
The world's largest vaccine manufacturer, the Serum Institute of India (SII), has sought the Drugs Controller General of India's (DCGI) permission to conduct a small domestic trial of the Novavax coronavirus vaccine that was found to be 89.3% effective in a UK trial, its Chief Executive Officer Adar Poonawalla told news agency Reuters on Friday
Earlier today, Novavax said that its Covid-19 vaccine was 89.3% effective in preventing the killer virus in a trial conducted in the United Kingdom, and was nearly as effective in protecting against the more highly contagious variant first discovered in Britain, as per a preliminary analysis.
Novavax is reportedly stockpiling Covid-19 vaccines at six operating manufacturing sites and said it expects a total of eight plants in seven countries to produce at the rate of 2 billion doses per year, including from the SII, a major player in the fight against coronavirus.
A mid-stage trial of its coronavirus vaccine in South Africa, where a troubling new variant of the deadly virus is common, showed 60% effectiveness among people who did not have HIV.
Last year in September, Novavax had announced its deal with the world's largest vaccine manufacturing firm SII in order to produce 2 billion doses of coronavirus vaccines.
As per reports, Novavax has said that it started making new versions of its Covid-19 vaccine to protect against emerging virus variants in early January and expects to select ideal candidates for a booster in the coming days.
The company also said that it plans to initiate clinical testing of these new vaccines in the second quarter of this year.
Novavax has also received around $388 million in backing from the Coalition for Epidemic Preparedness Innovation (CEPI), a Norway-based group backed by 14 governments, the Bill and Melinda Gates Foundation, and Britain’s Wellcome Trust.
Source - liveMint
ABOUT US
OUR COMPANIES
CORPORATE SOCIAL RESPONSIBILITY
© Copyrights 2026. Cyrus Poonawalla Group. All rights reserved.